Revolutionary Reversible Anticoagulation
AYA1809002 is a first-in-class, reversible DNA aptamer-based anticoagulant targeting thrombin with high affinity and tunable control via a sequence-paired antidote. It is engineered to provide safe, effective, and rapidly reversible anticoagulation for use in hospital/acute care settings and high-risk patient populations.
AYA1809002 is a first-in-class, reversible DNA aptamer-based anticoagulant targeting thrombin with high affinity and tunable control via a sequence-paired antidote. It is engineered to provide safe, effective, and rapidly reversible anticoagulation for use in hospital/acute care settings and high-risk patient populations.
Precision Targeting
500× selectivity for thrombin over other proteases with 10 nM binding affinity ensures effective inhibition in both fluid phase and clot-bound contexts.
Rapid Reversal
Sequence-paired DNA antidote binds 1:1 to fully restore Factor II activity in under 30 minutes – without foreign proteins or immune responses.
Superior Safety
≥24 hour blood stability at 37°C with minimal off-target effects, no CYP interactions, and no food/vitamin-K restrictions.
Engineered Design
500× selectivity for thrombin over other proteases with 10 nM binding affinity ensures effective inhibition in both fluid phase and clot-bound contexts.
Direct Thrombin Inhibition

Clinical Indications
- Non-valvular AF (stroke prophylaxis)
- Treatment/prevention of DVT/PE
- Acute coronary syndromes (adjunct)
- HIT

Mechanism & PK Advantages
- Competitive occupancy of thrombin’s catalytic cleft → blocks fibrinogen conversion & platelet activation
- Linear, predictable PK: t½ ~10 h, steady state in 2–3 days, QD/BID dosing

Our Aptamer’s Clearance
- Glomerular filtration only → rapid renal removal without active tubular secretion
- Stable in renal impairment (minimal protein binding)
- Versus FXa inhibitors: Xarelto ~33 % renal +CYP/P-gp (accumulates in CKD); Apixaban ~25 % renal + CYP3A4 interactions

Key Performance Metrics
- 500× selectivity for thrombin; 10 nM affinity; 24 h+ blood stability
- Dual activity: fluid-phase & clot-bound inhibition
Drug–Drug Interaction Profile
- No CYP or P-gp liability → avoids warfarin-like interactions
- No food/vitamin K restrictions
Inhibition of Clot-Bound Thrombin
AYA1809002 inhibits thrombin retained in fibrin clots. It binds to free and clot-bound thrombin, providing comprehensive anticoagulant activity.
Built-in Reversibility
Reversal Agent
Vitamin K / PCC
Protamine Sulfate
Idarucizumab
Modality
Cofactor ± concentrates
Polycation binds heparin
Humanized Fab for dabigatran
Key Limitations
Slow (6–24 h), thrombosis risk
Hypotension, anaphylaxis
$3 500+/vial, immunogenicity rebound risk
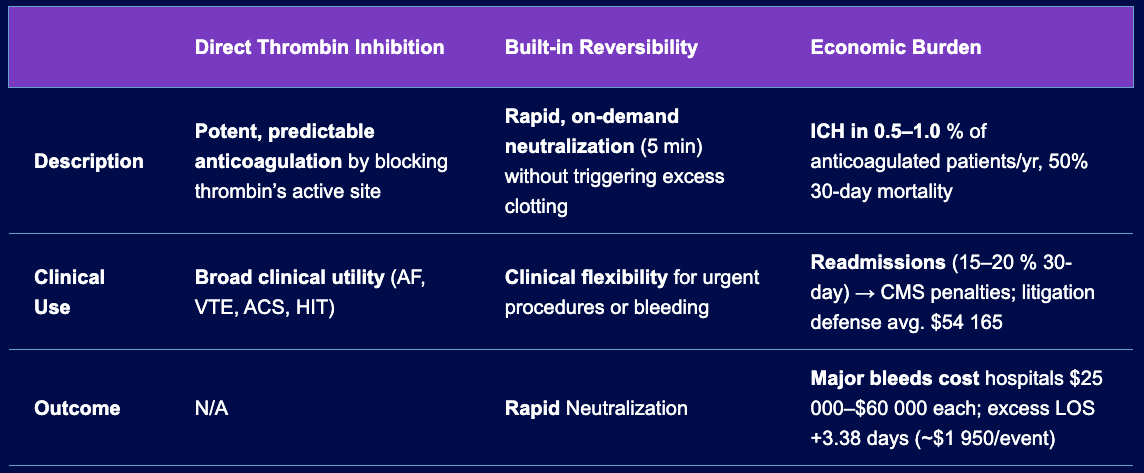
Comparison of Direct Thrombin Inhibition, Build in Reversibility, and Economic Burden
Comparison of Direct Thrombin Inhibition, Build in Reversibility, and Economic Burden
| Direct Thrombin Inhibition | Built-in Reversibility | Economic Burden | |
|---|---|---|---|
| Description | Potent, predictable anticoagulation by blocking thrombin’s active site | Rapid, on-demand neutralization (5 min) without triggering excess clotting | ICH in 0.5–1.0 % of anticoagulated patients/yr, 50% 30-day mortality |
| Clinical Use | Broad clinical utility (AF, VTE, ACS, HIT) | Clinical flexibility for urgent procedures or bleeding | Readmissions (15–20 % 30-day) → CMS penalties; litigation defense avg. $54 165 |
| Outcome | N/A | Rapid Neutralization | Major bleeds cost hospitals $25 000–$60 000 each; excess LOS +3.38 days (~$1 950/event) |
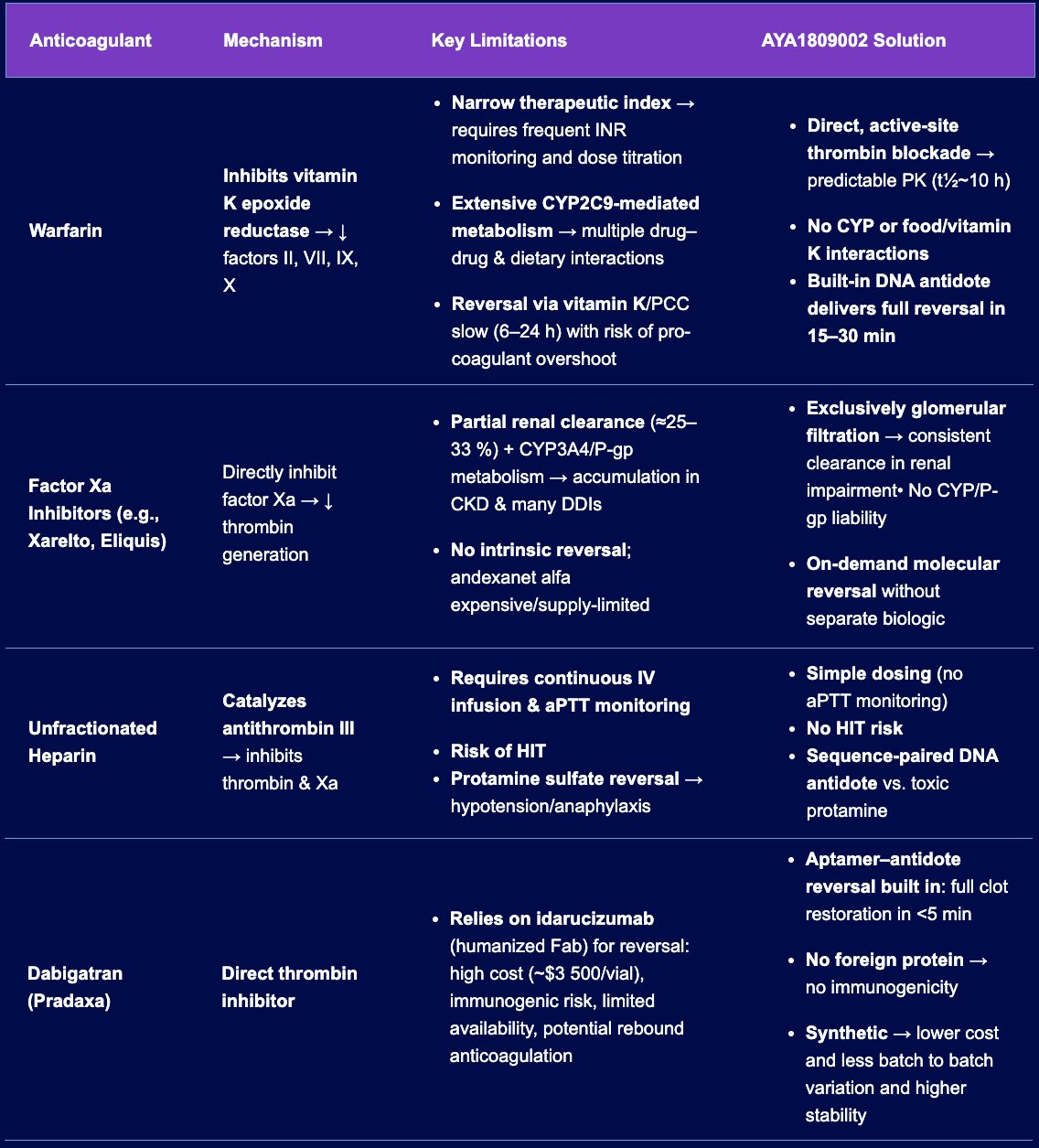
Comparison of Anticoagulants: Mechanisms, Limitations, and the AYA189002 Solution
Comparison of Anticoagulants: Mechanisms, Limitations, and the AYA189002 Solution
| Anticoagulant | Mechanism | Key Limitations | AYA1809002 Solution |
|---|---|---|---|
| Warfarin | Inhibits vitamin K epoxide reductase → ↓ factors II, VII, IX, X |
|
|
| Factor Xa Inhibitors (e.g., Xarelto, Eliquis) | Directly inhibit factor Xa → ↓ thrombin generation |
|
|
| Unfractionated Heparin | Catalyzes antithrombin III → inhibits thrombin & Xa |
|
|
| Dabigatran (Pradaxa) | Direct thrombin inhibitor |
|
|
AYA1809002 Development Timeline (Projected)
Leadership & Scientific Team Bios
AYA1809002 Program

AyassBioScience, LLC Company Overview

Dr. Mohamad Ayass, MD
President and CEO, Ayass Bioscience

Dr. Lina Abi Mosleh, PhD
Vice President & Principal Scientist

Kevin Zhu, MS
Bioinformatics Scientist

Dr. Natalya Griko, PhD
Senior Scientist, Aptamer Development

Dr. Victor Pashkov, PhD
Research Scientist, Molecular Biology

Dr. Jin Zhang, MD, MPH
Clinical Epidemiologist


