Bridging Science and Precision Patient Care
Ayass BioScience, LLC (DBA as Ayass Laboratory, LLC) is a CLIA certified laboratory offers early disease detection, real-time gene expression analysis, and intelligent diagnostics that go beyond trial-and-error approaches. Ayass BioScience integrates Agentic AI, Transcriptome Analysis, and Aptamer Technology to advance precision medicine. With cutting-edge molecular testing and a dedicated bioinformatics team, we help clinicians make personalized, proactive treatment decisions. Discover a new era of data-driven healthcare at Ayass BioScience, LLC.
Advanced Next Generation Sequencing (Genetic Testing). Cardiac Conditions Hereditary Risk Assessment, Hereditary Cancer Risk Assessment, Pharmacogenetic Testing, Cytokine Testing and Innovative Laboratory Testing. Ayass BioiScience, LLC is working on Aptamers Research and Testing. Transcriptome Analysis is conducted at Ayass BioScience, LLC for research purposes only and is not FDA approved. Our Scientific Publications.
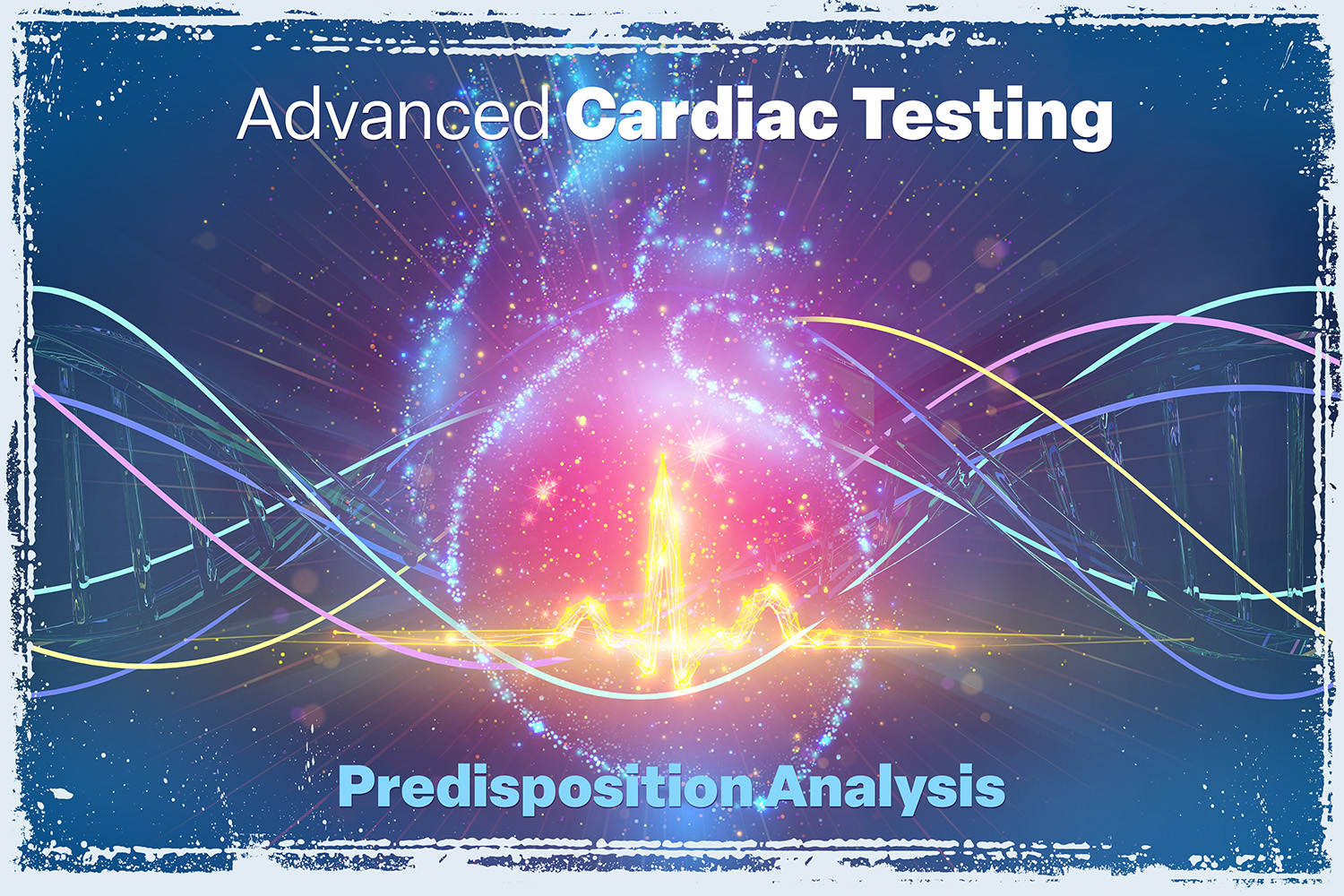
Genetic Testing – Heart Diseases
Understand Your Genetic Risk for Heart Disease — With Precision. Comprehensive analysis of 174 genes linked to 17 cardiovascular disorders. READ MORE.
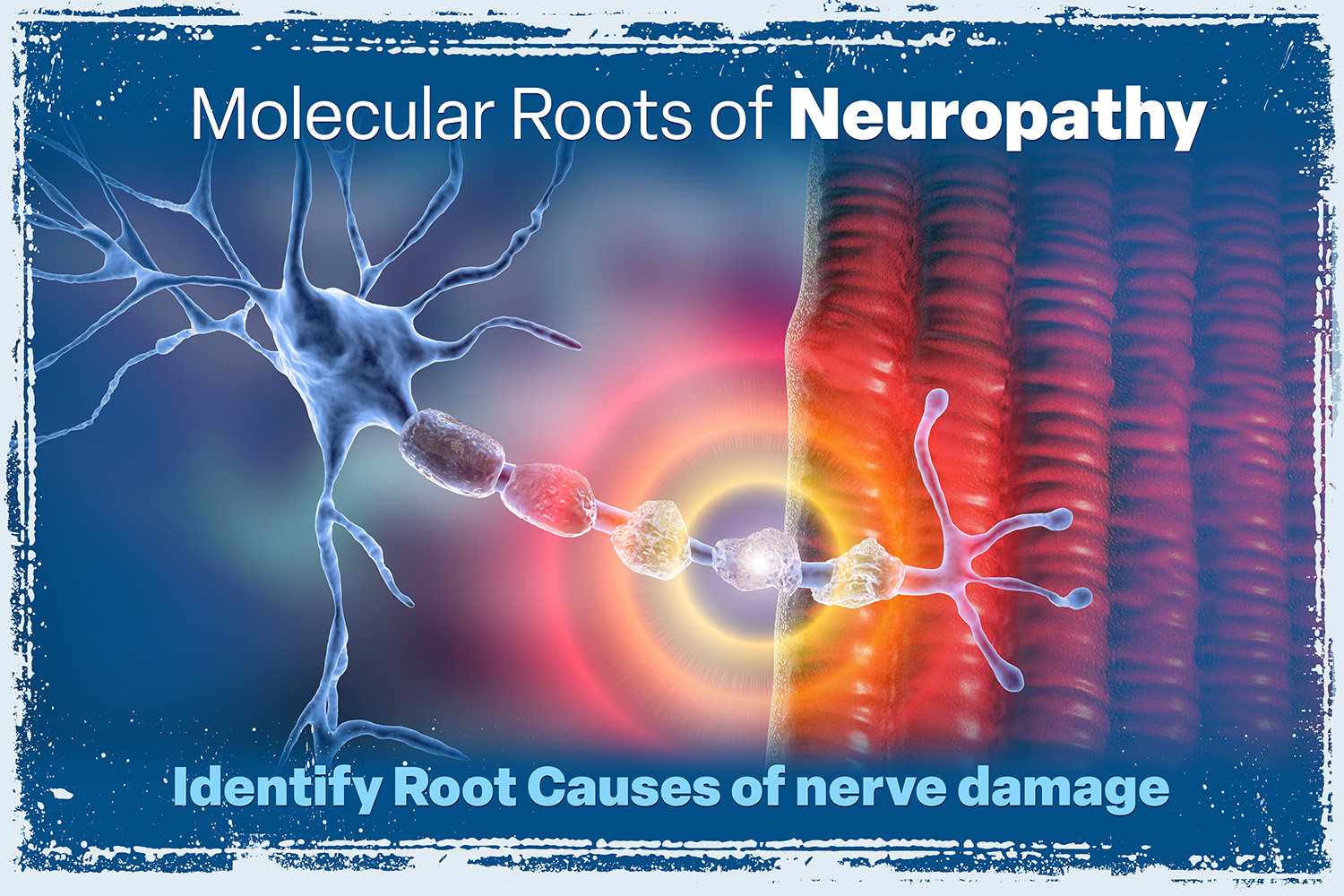
Transcriptomic Neuropathy Analysis
Exploring the Molecular Roots of Your Neuropathy. Our test analyzes gene expression in several vital areas that offer deeper insight into the biological mechanisms behind neuropathy. LEARN MORE
Obesity Management
Our comprehensive transcriptomic analysis evaluates genes related to metabolism, hunger control, fat accumulation, insulin response, inflammation, and how your body expends energy. LEARN MORE
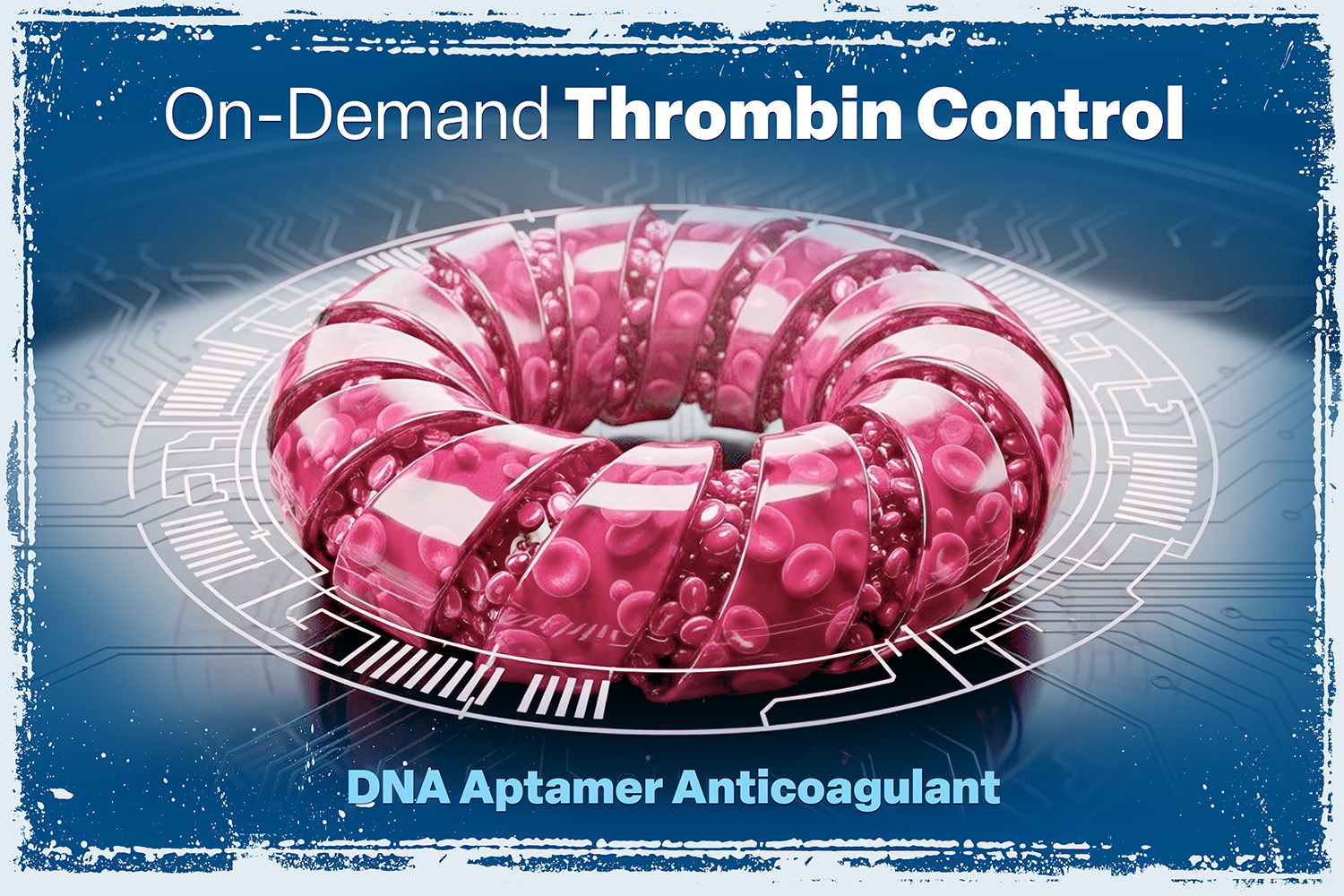
DNA Aptamer Anticoagulant
A first-in-class, reversible DNA aptamer-based anticoagulant targeting thrombin with high affinity and tunable control via a sequence-paired antidote. READ MORE.
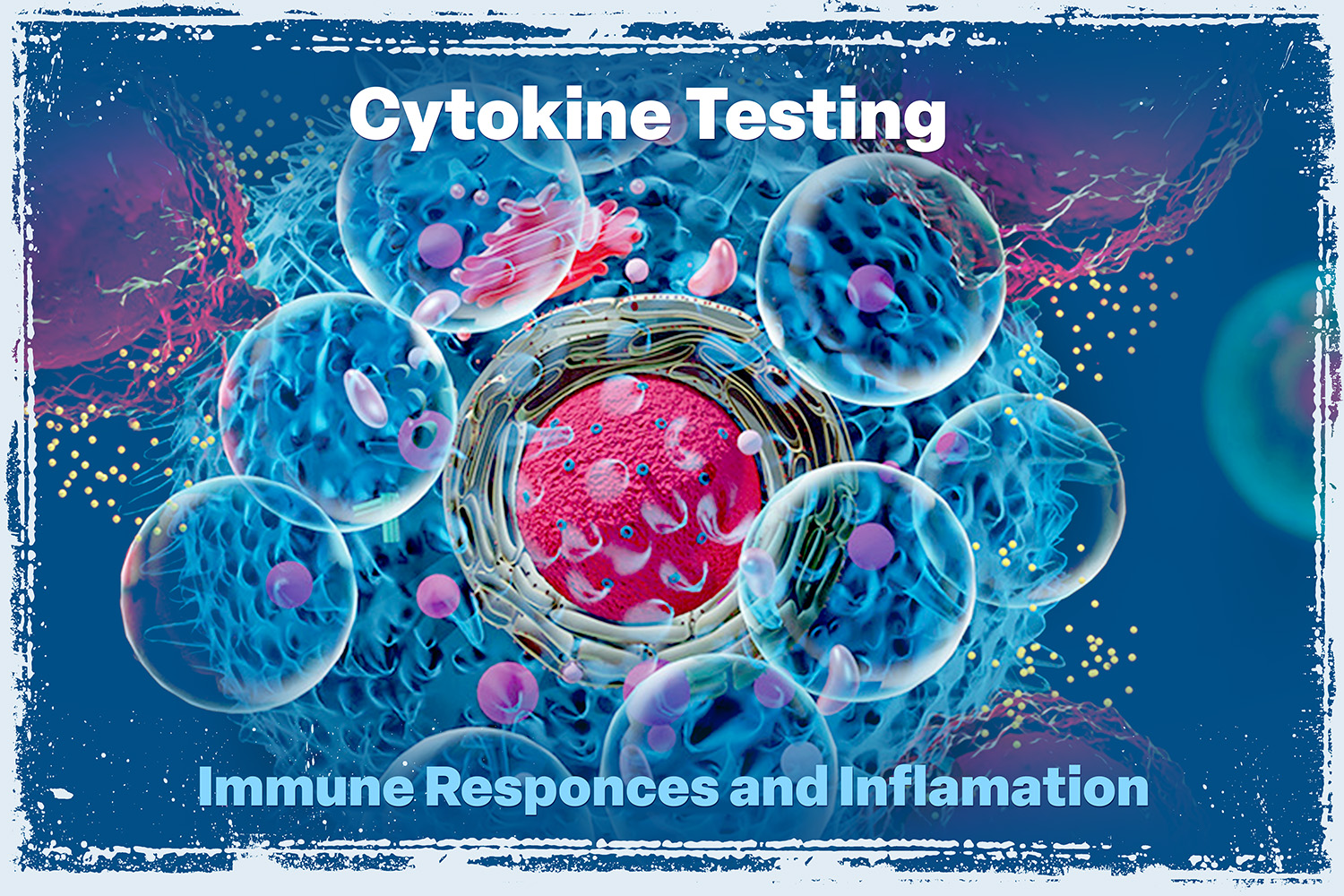
Cytokine Testing
Cytokine testing is a medical diagnostic technique used to measure the levels of various cytokines in a patient’s blood to understand Immune Responses and Inflammatory Processes. LEARN MORE
All tests are performed at Ayass BioScience, LLC (DBA Ayass Laboratory, LLC), a CLIA-certified laboratory.
If you have any questions about Genetic Testing, Clinical Testing, Cytokine Testing, or Pharmacogenetic Testing at Ayass BioScience, LLC—or about Transcriptome Analysis (conducted at Ayass BioScience, LLC for research purposes only and not FDA-approved)—please call us at 972-668-6005 or fill out the contact form at the bottom of this page. We’ll be happy to answer your questions.
Ayass BioScience, LLC website is secured.
All transactions on this site are protected with high grade SHA-2 and 4096-bit encryption.