Respiratory Pathogens Collection
USING NASOPHARYNGEAL SWABS
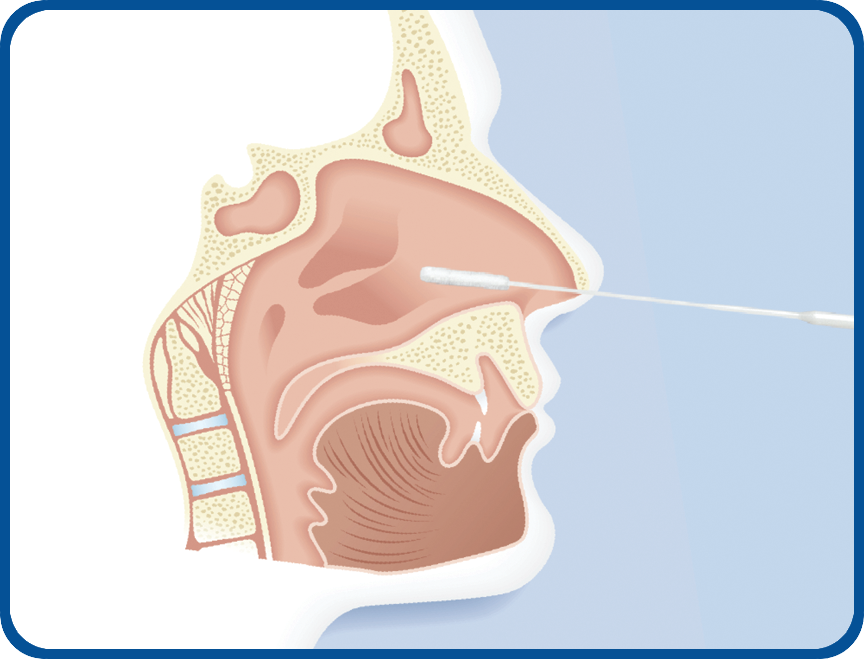
Nasopharyngeal swabs are used for the detection of respiratory pathogens such as RSV, influenza virus A & B or parainfluenza virus.
A properly collected viral swab (one nare (a single nostril) is sufficient) is necessary for detection of these organisms. After collection, the specimen is stored in a buffer and ships to the lab.
1) Tilt patient’s head back 70 degrees.
2) Insert swab into nostril (swab should reach depth equal to distance from nostrils to outer opening of the ear.) Leave swab in place for several seconds to absorb secretions.
3) Slowly remove swab while rotating it (swab both nostrils with same swab).
4) Place tip of swab into sterile viral transport media tube and snap/cut off the applicator stick.
Specimen Rejection Criteria
In order for Ayass Bioscience, LLC laboratory to be compliant and to provide the most reliable patient results possible, all personnel must adhere to strict guidelines for accepting patient specimens and requisitions. Specimens will be evaluated for adequacy by staff throughout the testing process. Inadequate or unsuitable specimens will need to be re-collected in order to be processed. Below is a list of cases where a specimen will be rejected and therefore NOT be processed for SARS CoV-2 Real Time RT-PCR.
Specimens may be deemed unacceptable for the following reasons:
1. Unlabeled, illegibly labeled, mislabeled, or inadequately labeled specimens. Specifically each specimen should have patient identifiers:
Patient Name, Date of Birth, Sample Collection Date and Time.
- Samples that require minimal additional information but are otherwise deemed suitable may only be processed following the adequate verification process as described above.
2. Specimens received with incomplete requisition form information or without a requisition form.
3. Discrepancies between specimen label and requisition form information.
4. Incorrect container for test requested. Samples must be collected using VTM provided by Ayass BioScience, LLC.
5. Visibly contaminated, leaking, damaged or inappropriate collection container.
6. Contaminated requisition forms.
7. Specimen received at temperatures outside of the acceptable range. Specimen should be refrigerated (2-8°C) or frozen (below -20°C).
8. Specimen sent after 6 days of sample collection. Any sample received after that time will NOT be processed for SARS-CoV-2 Real Time RT-PCR testing.
Resolution of Discrepancies:
1. Every attempt will be made to resolve discrepancies due to requisition or specimen labeling with the ordering practitioner in a timely manner before testing is to proceed.
2. Sample integrity will be maintained during the holding period.
If resolution is delayed and the integrity of the sample will be compromised, the sample may, at the discretion of the laboratory, be processed with a comment documenting the discrepancy and results may be held until the discrepancy has been resolved.

Saliva Cell Collection
ORAcollect.Dx is intended for use in the non-invasive collection of saliva samples.
Human DNA from the saliva sample is isolated, stabilized and is suitable for use in molecular diagnostic applications.
1. Refrain from eating, smoking, or chewing for at least 30 minutes before sample collection.
2. Donors with xerostomia (dry mouth) may not collect adequate sample using these instructions resulting in low yield DNA. In that case DNA extracted from whole blood is recommended.
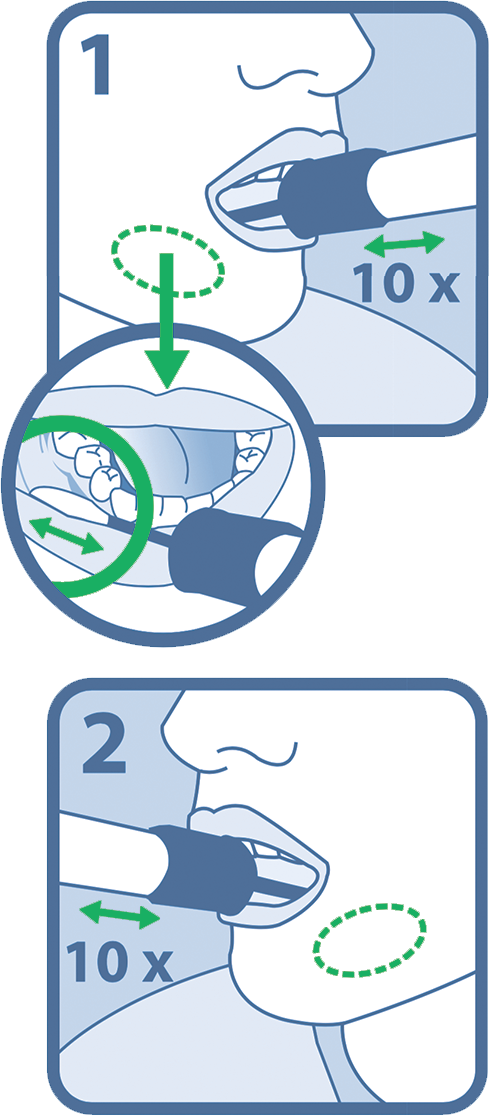
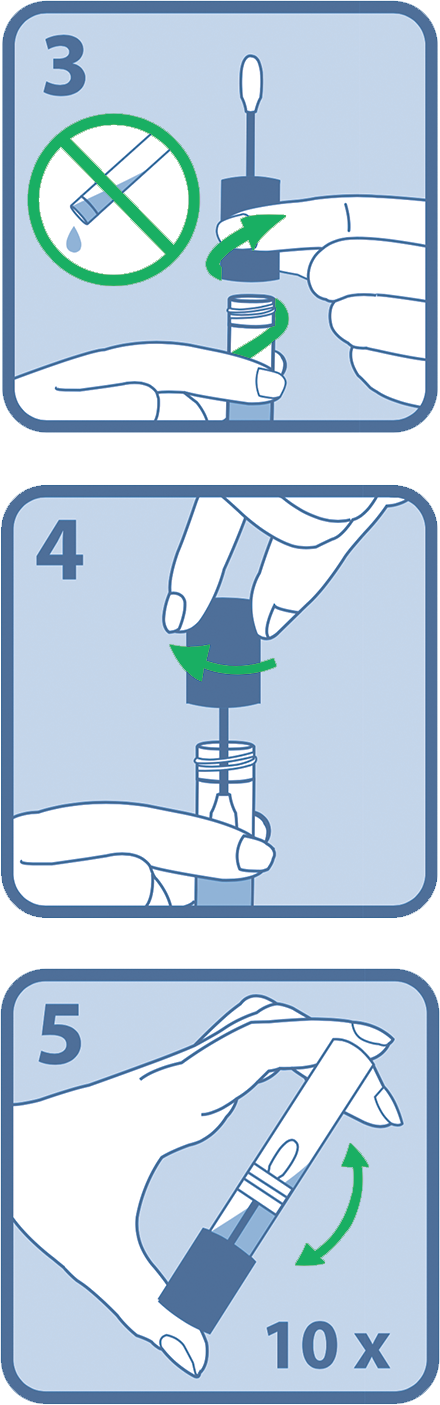 3. Open package from the end with the ‘open here arrow’.
3. Open package from the end with the ‘open here arrow’.
4. Remove collector by its handle from the package while ensuring that the sponge tip does not come in contact with any surface prior to collection.
5. Place sponge as far back in the mouth as comfortable (Figure 2).
6. Gently rub along lower gum in a back and forth motion 10 times. Avoid rubbing the teeth.
7. Choking Hazard: Caution must be taken when inserting the sponge into the donor’s mouth.
8. Repeat rubbing motion on opposite side along the lower gum for another 10 times.
9. Hold the tube with the blue liquid upright to prevent it from spilling.
10. Unscrew the blue cap from the tube without touching the sponge (Fugure 3).
11. Turn the cap upside down and insert the sponge into the tube and close the cap tightly (Figure 4).
12. Invert capped tube and shake vigorously 10 times (Figure 5).
Specimen Rejection Criteria
In order for Ayass Bioscience, LLC laboratory to be compliant and to provide the most reliable patient results possible, all personnel
must adhere to strict guidelines for accepting patient specimens and requisitions. Specimens will be evaluated for adequacy by staff throughout the testing process. Inadequate or unsuitable specimens will need to be re-collected in order to be processed. Below is a list of cases where a specimen will be rejected and therefore NOT be processed for genetic testing:
Specimens may be deemed unacceptable for the following reasons:
1. Unlabeled, illegibly labeled, mislabeled, or inadequately labeled specimens. Specifically each specimen should have patient identifiers:
Patient Name, Date of Birth, Sample Collection Date and Time.
- Samples that require minimal additional information but are otherwise deemed suitable may only be processed following the adequate verification process as described above.
2. Specimens received with incomplete requisition form information or without a requisition form.
4. Discrepancies between specimen label and requisition form information.
5. Incorrect container for test requested.
6. Visibly contaminated, leaking, damaged or inappropriate collection container.
7. Contaminated requisition forms.
8. Specimen received at temperatures outside of the acceptable range.
9. Specimen sent after 15 days of sample collection. Any sample received after that time will NOT be processed for Genetic Testing.
Resolution of Discrepancies:
1. Every attempt will be made to resolve discrepancies due to requisition or specimen labeling with the ordering practitioner in a timely manner before testing is to proceed.
2. Sample integrity will be maintained during the holding period.
If resolution is delayed and the integrity of the sample will be compromised, the sample may, at the discrection of the laboratory, be processed with a comment documenting the discrepancy and results may be held until the discrepancy has been resolved.

Human Peripheral Whole Blood
Collection Procedure for Human Peripheral Whole Blood Specimen:
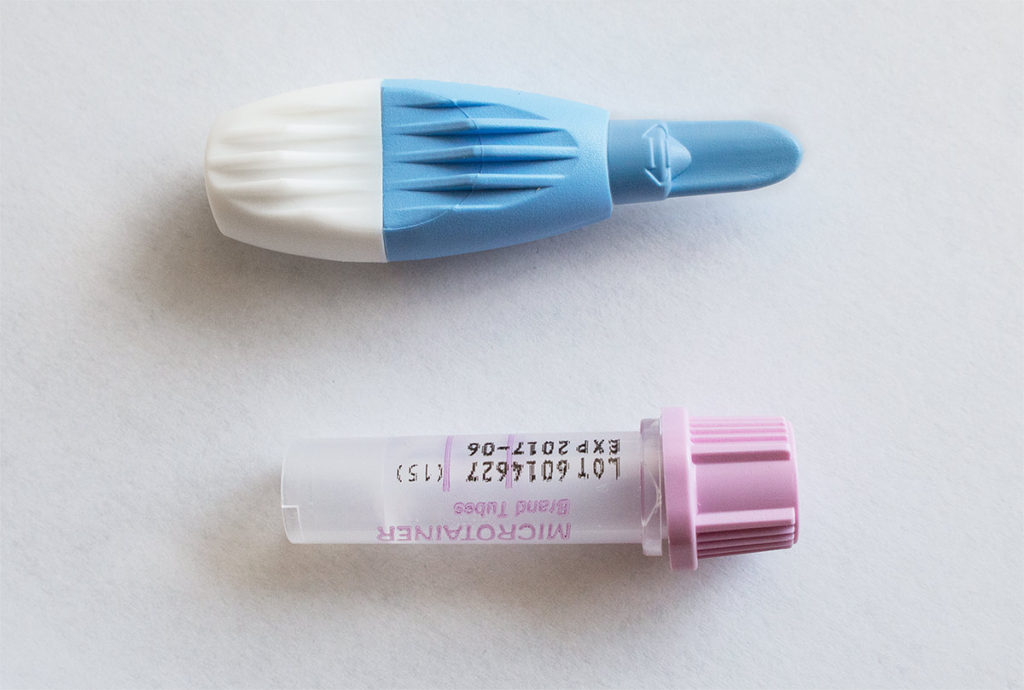
When you submit an online request, you will receive a parcel by mail that will include the following items:
1. One Touch Activated Lancet
2. One EDTA (Lavender Top) microtainer tube
3. Documents that contain instructions for sample collection, customer information and genetic testing consent form
Please follow the simple outlined steps for sample collection:
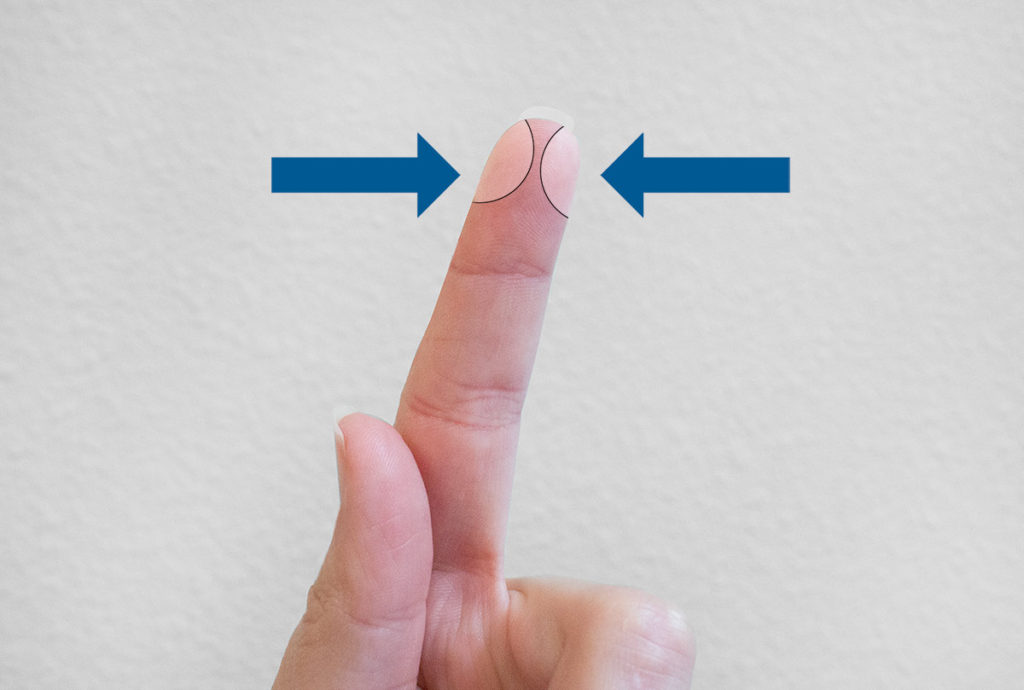
Wash your hands with soap and water.
Identify the puncture site.

Twist off the tab on the lancet to break the seal.

Discard the tab.

Position the safety lancet firmly against the puncture site.

Press the lancet firmly against the puncture site. You should hear an audible click! Do not remove the device from the site until an audible click is heard.

The blade will safely retract once the lancet is removed from the puncture site. Discard the used lancet.

Open the microtainer tube. Apply pressure on the puncture area and fill the microtainer tube with blood. It is very important for the blood to flow into the microtainer tube. Do not scrape the puncture area against the side of the microtainer tube. This will affect the quality of the genomic DNA obtained.
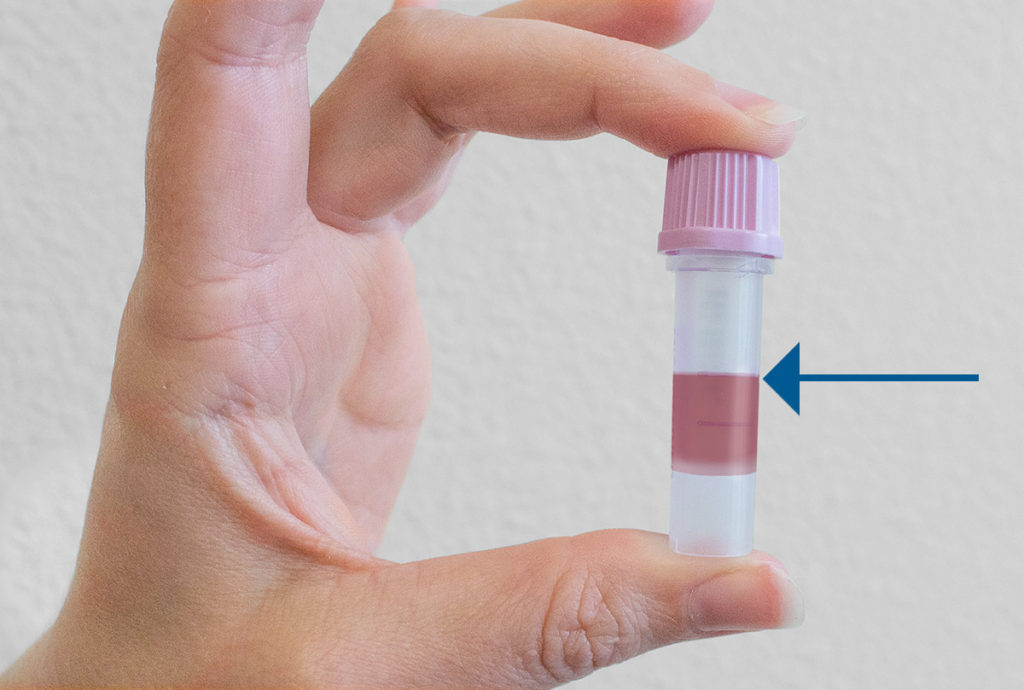
Fill the microtainer tube with blood between the 250-500 microliters marks showing on the tube.
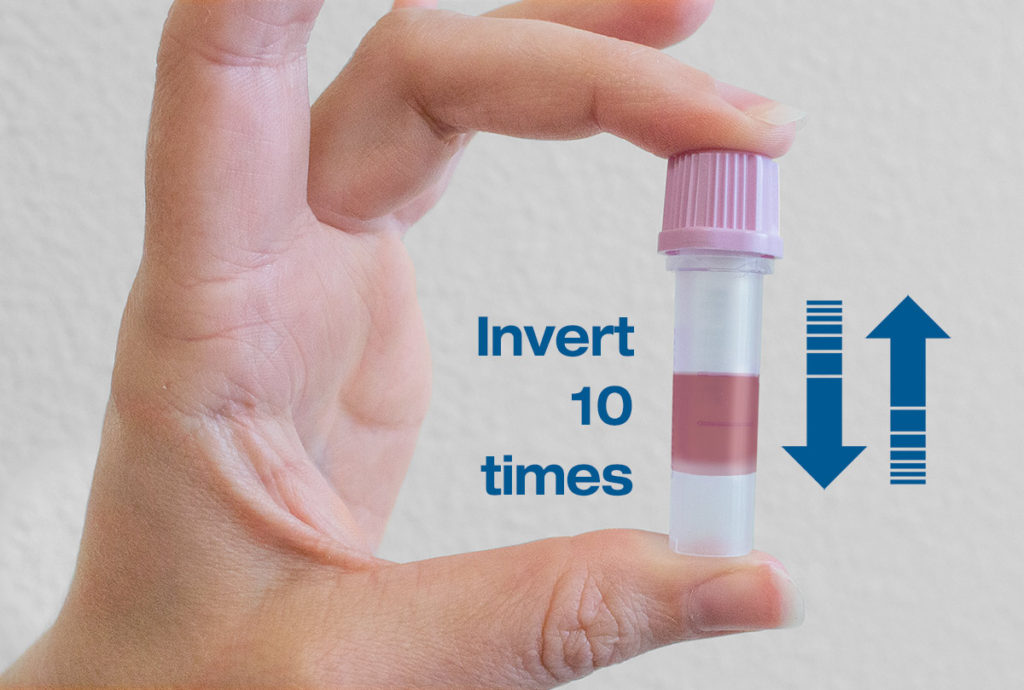
Close the lid firmly and invert the microtainer tube gently ten times to mix the blood with EDTA (Anticoagulant).
Label the tube with your name, date of birth and collection date. To obtain optimal results, the samples must be shipped the same day the blood specimen is collected. The pre-paid parcel is intended for overnight delivery at ambient temperature with arrival Monday-Friday during normal business hours. Alternatively, the blood can be refrigerated up to 3 days and shipped overnight at ambient temperature. Blood specimen that is shipped later will be tested and will only be processed if the genomic DNA quality and quantity permits. In hot weather a cool pack can be enclosed.
A blood sample will be rejected if: It is frozen or partially frozen. It is hemolyzed or clotted. Blood sample is collected from a patient who has undergone a heterologous bone marrow transplant.
Unless requesting the ctDNA genetic testing, we highly discourage the submission of samples from patients who have active hematological malignancies. If such patients are interested in the other panels offered at Ayass BioScience, LLC we recommend using genomic DNA sample extracted from a skin biopsy.
If you have any questions about Specimen Collection for Testing at Ayass BioScience, LLC, please call today at 972-668-6005 or fill out our contact form on the bottom of this page. We will answer any question you might have.