Exploring the Molecular Roots of Your Neuropathy
A closer look at the advanced science behind the Ayass Bioscience, LLC Neuropathy Test using Autonomous Biomarker Discovery Engine
Discover the Science Behind the Ayass Bioscience, LLC Neuropathy Analysis
For questions about Neuropathy Analysis using Autonomous Biomarker Discovery Engine and next steps, contact us at 972-430-6872. If you reach us after hours, just leave a message and we will return your call.
Welcome to a deeper look into the innovative science that powers the Ayass Bioscience, LLC Neuropathy Test
This page explores the complex cellular systems, molecular components, and biological pathways analyzed through our advanced transcriptomic approach. By examining gene activity at this level, we can uncover the unique molecular patterns that contribute to each individual’s experience of neuropathy.
Our detailed analysis focuses on transcriptomic markers associated with Neurons, Schwann cells, Microglia, Fibroblasts, Endothelial cells, and Satellite Glial cells—all examined through PBMCs (Peripheral Blood Mononuclear Cells). These immune cells provide insight into the intricate pathophysiology of peripheral neuropathy.
Changes in these markers can reflect both the progression of neuropathic sensory and motor dysfunction, as well as the body’s potential for nerve repair.
At the core of this powerful process is the Ayass Bioscience, LLC, where your sample is carefully processed and analyzed. The resulting data is interpreted using our Proprietary Autonomous Biomarker Discovery Engine – a sophisticated artificial intelligence system developed in-house by Ayass Bioscience, LLC.
By combining high-precision lab work with advanced AI interpretation, we deliver an unmatched view of your molecular health.
Explore Key Molecular Pathways
Our test analyzes gene expression in several vital areas that offer deeper insight into the biological mechanisms behind neuropathy. Click on each category to learn more:
Molecular Targets & Neuropathy
Understanding the Broader Context
While the Ayass BioScience, LLC Neuropathy Test maps your individual gene expression patterns across key cellular systems, it also provides valuable insight when viewed in the context of well-established molecular targets and pathways studied in neuropathic pain research.
Our comprehensive analysis may uncover dysregulation in genes associated with:
Identifying whether genes related to these targets are differentially expressed in your profile can offer meaningful insights and support more informed discussions with your healthcare provider about the underlying mechanisms of your neuropathy.

Disclaimer: This information is provided for general educational purposes only. The Ayass Bioscience, LLC Neuropathy Test does not diagnose specific conditions or recommend particular treatments. All medical decisions should be made in consultation with a qualified healthcare provider who can assess your full medical history and other diagnostic findings.
To offer a more complete understanding, it’s helpful to consider the current strategies used in managing neuropathic pain:
Research is active in areas like selective Nav channel blockers (e.g., Nav1.7, Nav1.8 inhibitors), HCN channel blockers, AAK1 inhibitors, new drug delivery systems, and therapies targeting specific inflammatory or neurotrophic pathways.
For questions about Neuropathy Analysis using Autonomous Biomarker Discovery Engine and next steps, contact us at 972-430-6872. If you reach us after hours, just leave a message and we will return your call.
Beyond the Surface: Your Path to Molecular Insight
The Ayass BioScience, LLC Neuropathy Test, powered by comprehensive transcriptome analysis and the integrative strength of our proprietary Autonomous Biomarker Discovery Engine, offers a deep exploration into the complex molecular symphony — or discord — occurring within your nervous system.
By analyzing key cellular components, pathways, and their interactions, this test provides you and your healthcare provider with profound insights. This detailed molecular blueprint becomes a powerful tool for understanding the unique nature of your neuropathy, helping support more informed conversations and personalized care strategies on your health journey.
Beyond Symptoms: Gain Unprecedented Insight into Your Neuropathy’s Molecular Roots
Looking Deeper: A New Approach to Understanding Neuropathy
Are you navigating the challenging path of neuropathy, searching for more than just surface-level answers?
We offer an advanced testing service designed to uncover the deep biological drivers behind neuropathy — not just symptoms. This isn’t just another test. It’s a molecular-level investigation into the unique gene activity within your body that may be contributing to nerve dysfunction, pain, or impaired healing.
A Molecular Blueprint of Nerve Health
Using high-resolution transcriptome analysis, our test expands upon key markers related to neurons, Schwann cells, microglia, fibroblasts, endothelial cells, and satellite glial cells — all analyzed through PBMCs (peripheral blood mononuclear cells).
Changes in these immune cells reflect the complex biology of peripheral neuropathy. These cellular players are deeply involved not only in the development of sensory and motor dysfunction, but also in the body’s potential for nerve repair — and their status can now be monitored through your blood.
Unparalleled Depth. Just One Sample.
With just a small blood sample (approx. 1ml from fingerstick or 4ml from venous draw), your test begins at the Ayass BioScience Transcriptome Data Center (ABS TDC) in Dallas–Fort Worth.
There, our proprietary Agentic AI platform begins analyzing over 200 million potential pathway combinations, uniquely relevant to your nervous system.
This extraordinary depth uncovers molecular patterns, connections, and disruptions that traditional nerve tests simply can’t detect.
The Neuropathy Enigma: Why Deeper Cellular Understanding is Key
Neuropathy is deeply personal, rooted in the intricate fabric of your cellular and molecular biology.
Standard diagnostic tests often only scratch the surface. To achieve a truly comprehensive understanding, we must look deeper — into the inner workings of critical biological systems.
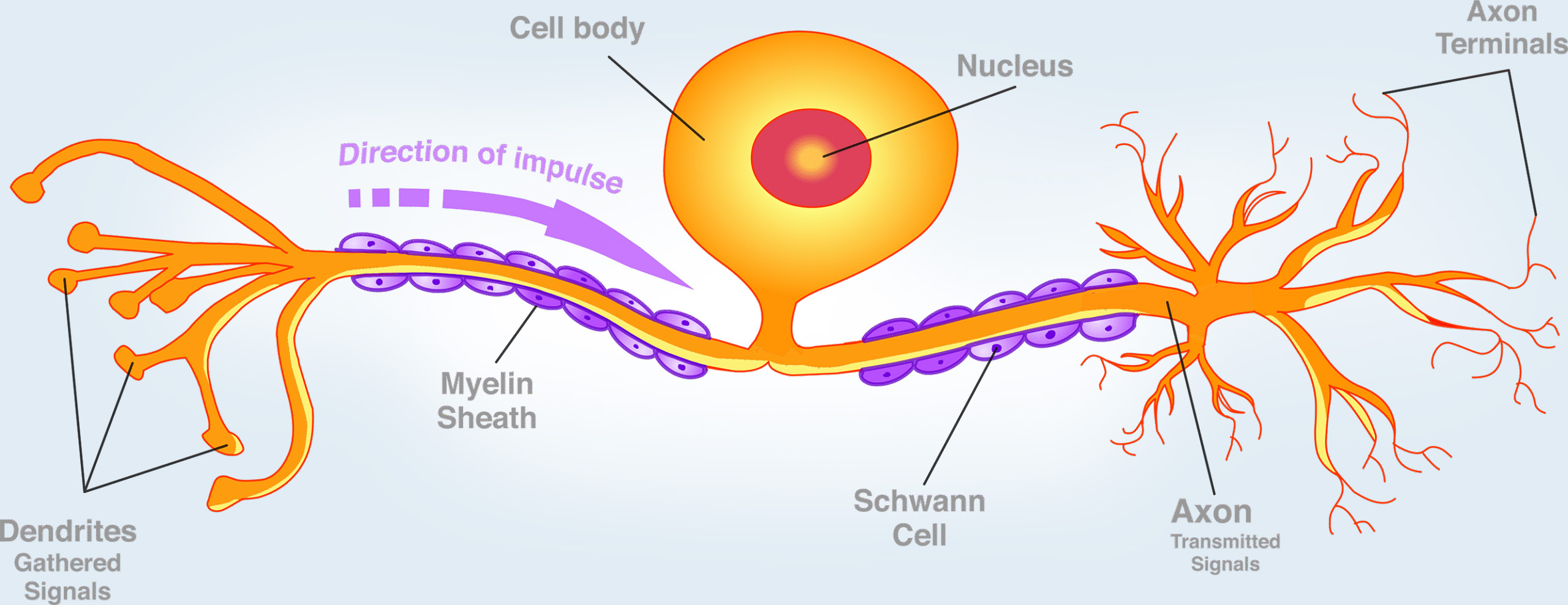
This includes a vast array of neural cell types, from motor and sensory neurons to glial cells such as Schwann cells, oligodendrocytes, astrocytes, and microglia. It also involves their complex subcomponents, including axonal transport systems, myelin sheaths, and synaptic dynamics.
Layered on top are numerous dynamic molecular pathways — from neuroinflammation and oxidative stress to neurotrophic factor signaling — all of which may contribute to the development or progression of neuropathy.
Our advanced analysis is designed to do exactly that. We investigate:
Crucially, these multifaceted biological insights — spanning cellular components, their substructures, and dynamic molecular pathways — are meticulously analyzed and integrated through our proprietary Autonomous Biomarker Discovery Engine.
This sophisticated integration, developed in-house at Ayass BioScience, LLC, is what truly sets our analysis apart. It enables a level of molecular precision and contextual understanding that is genuinely cutting-edge, offering insights far beyond the reach of conventional diagnostic tools.
Autonomous Biomarker Discovery Engine Analysis: Revealing Your Active Neural Instructions
Our Process
The Ayass BioScience, LLC Difference
Your investment in our Neuropathy Transcriptome Analysis delivers extraordinary and unprecedented worth because it provides:
Important Information
Please note: Our molecular testing is currently designated for Research Use Only (RUO) and has not yet received FDA approval.
What You Receive with Your Order

-
Blood collection kit with prepaid return shipping
(Choose from finger prick or venipuncture options) -
Full RNA sequencing focused on metabolism-related gene expression
-
Advanced analysis via our proprietary Autonomous Biomarker Discovery Engine
-
A comprehensive, personalized metabolic assessment report
Peripheral Neuropathy and PBMC Autonomous Biomarker Discovery Engine Analysis
Blood cells can reveal nerve damage occurring in distant locations. This groundbreaking approach from Ayass Bioscience, LLC uses PBMC transcriptome signatures to understand changes in the peripheral nervous system.
Core Concept
Transcriptome Signatures
PBMCs reflect changes happening in the peripheral nervous system.
Blood as a Window
Blood cells provide insights into nerve damage occurring in distant locations.
Diagnostic Potential
This approach may revolutionize how we detect and treat neuropathy.
Cellular Mechanisms in Neuropathic Pain: A Multi-Cell Perspective
Exploring key cellular players in neuropathic pain pathology and emerging therapeutic avenues.
Sensory Neurons: Hyperexcitability and Ion Channels
Membrane Hyperexcitability
Spontaneous firing and reduced thresholds
Ion Channel Dysregulation
Nav1.7/1.8/1.9 altered expression
Therapeutic Targets
Selective Nav blockers under development
Schwann Cells: From Support to Inflammation
Myelinating State
Normal supportive function
Dedifferentiation
P75 and GFAP upregulation
Pro-inflammatory
Cytokine production increases
Therapeutic Target
Remyelination promoters
Immune Cells: Inflammatory Mediators
Macrophages
M1 phenotype predominates in pain states
Microglia
Central sensitization contributors
T Lymphocytes
Sex-specific pain mechanisms
Therapeutic Approaches
Immune modulators show promise
Fibroblasts: Matrix Remodeling and Fibrosis
Activation Phase
Transformation to myofibroblasts
Remodeling Phase
Excessive ECM production
Fibrotic Phase
Nerve compression and ischemia
Therapeutic Targets
Anti-fibrotic agents under investigation
Endothelial Cells: Blood-Nerve Barrier Disruption
Tight Junction Loss
Claudin-5 and ZO-1 downregulation
Increased Permeability
Inflammatory mediator infiltration
Vascular Remodeling
Microvascular changes worsen perfusion
Therapeutic Approach
Barrier stabilizers show potential
Satellite Glial Cells: Activation in Pain States
Activation Markers
GFAP upregulation indicates reactivity
Altered Gap Junctions
Increased coupling between cells
Purinergic Signaling
P2X7 receptor expression increases
Therapeutic Potential
Gap junction blockers reduce pain signaling
Cellular Crosstalk: Integrated Pain Pathways
Sensory Neurons
Release neuropeptides activating adjacent cells
Vascular Components
Facilitate infiltration of blood-borne factors
Schwann Cells
Respond with cytokine production
Immune Cells
Amplify inflammatory cascade
Sex Differences: Cellular Mechanisms
Male-predominant Mechanisms
Microglia-mediated pathways:
- P2X4R signaling critical
- TLR4 activation prominent
- BDNF release mechanism
Female-predominant Mechanisms
T-cell mediated responses:
- Adaptive immunity involvement
- Estrogen modulation effects
- Distinct cytokine profiles
Temporal Dynamics: Acute vs. Chronic Pain
Acute Phase (Days 1-7)
- Wallerian degeneration
- Robust macrophage infiltration
- Immediate Schwann cell dedifferentiation
Subacute Phase (Weeks 1-6)
- T-cell recruitment increases
- Satellite glial cell activation peaks
- Fibroblast activation accelerates
Chronic Phase (Months+)
- Persistent ion channel remodeling
- Established fibrotic changes
- Maladaptive neuronal plasticity
Therapeutic Landscape: Multi-cellular Targets
Emerging therapeutics based on cellular mechanisms target multiple pain pathways simultaneously.
Future Directions: Precision Medicine Approaches
Genetic Profiling
Ion channel variants predict drug response
Cellular Biomarkers
Personalized pain mechanism identification
Combinatorial Therapies
Multi-cell targeted approaches
AI-Guided Decisions
Treatment algorithms predict outcomes



